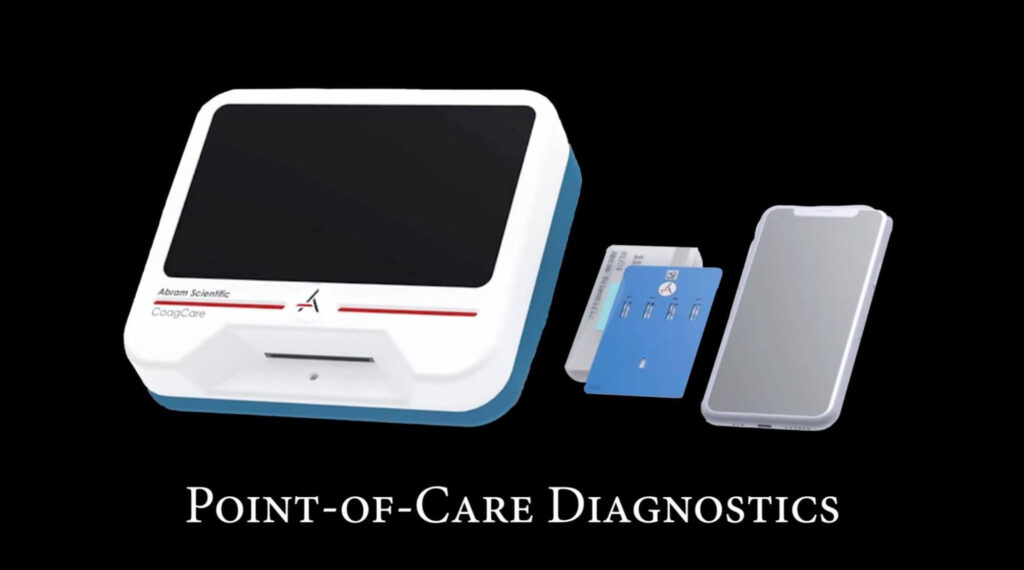
Abram Scientific is on a mission to revolutionize point-of-care diagnostic systems. As a fast-moving medical technology startup, they are building innovative products designed to deliver rapid, accurate results in clinical and remote environments.
In the fast-paced world of medical diagnostics, especially in areas as vital as blood management, startups face a difficult yet defining decision early in their journey: how to build the right digital infrastructure to support product development.
From the outset, it was clear we needed a modern, flexible, and integrated solution to manage our product data. After years of experience with traditional PLM systems, including Arena, we found what we were looking for in OpenBOM—a cloud-native, CAD-integrated platform that redefines what’s possible for startups building complex medical devices.
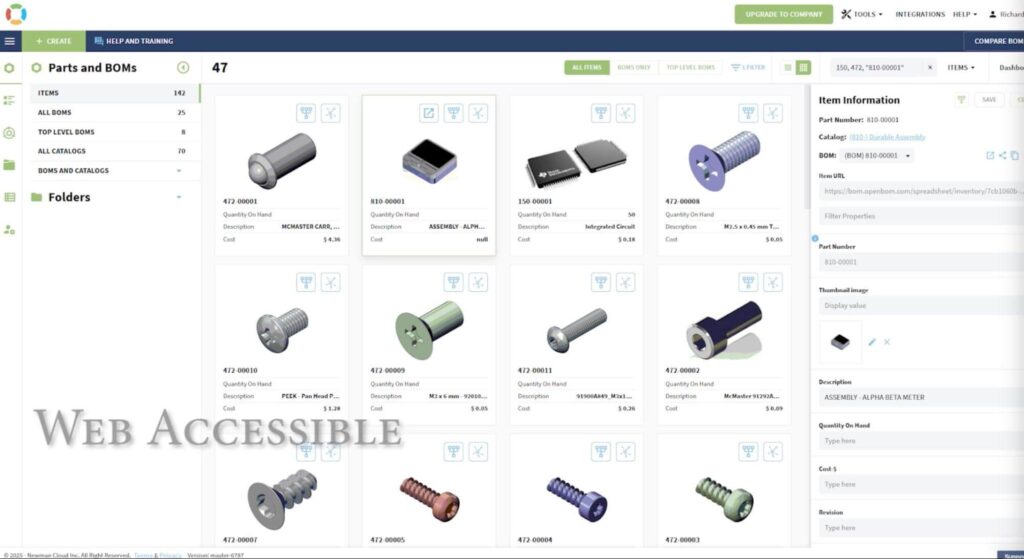
The Challenge: Outdated PLM Systems and Spreadsheet Chaos
Startups in regulated industries like medical devices often begin with the simplest tools available—spreadsheets. But what starts as a quick solution soon becomes an unmanageable tangle of data silos, broken formulas, and versioning nightmares.
But as VP of Engineering Richard Wiard knows from experience, building breakthrough medical products isn’t just about smart engineering—it’s about managing data with precision, consistency, and traceability from day one.
“Many early-stage medical companies get stuck in spreadsheets,” says Richard. “It feels like you’re moving quickly, but in reality, you’re losing control—versioning breaks down, collaboration suffers, and it gets harder to trust your data.”
Richard continues, “I personally administered Arena PLM for over eight years. While it was once the gold standard for early-stage companies, it hasn’t kept up with the demands of today’s agile development workflows. It was difficult to scale, required significant overhead, and lacked real-time integration with the tools we use daily.
The more complex our products became, the more it became clear: we needed a system that didn’t just store data, but worked with us to manage it.

With years of experience using traditional PLM platforms like Arena Solutions, Richard recognized the limits of legacy tools.

Discovering OpenBOM: A Purpose-Built Solution for Modern Development
We discovered OpenBOM during a critical phase of our development cycle. From day one, it stood out as a truly modern alternative. OpenBOM doesn’t treat product data as a static archive—it treats it as a living, connected resource.
One of the most powerful capabilities for us is OpenBOM’s seamless CAD integration. Whether we’re using mechanical CAD (MCAD) or electronic CAD (ECAD), OpenBOM automatically pulls design data and builds a structured, multi-level BOM that reflects exactly what we’re designing.
No more manual entry. No more mismatched versions. No more copying and pasting between systems. That’s a major leap forward when your team is juggling tight timelines and strict compliance requirements.
Richard chose OpenBOM as the foundation for Abram Scientific’s product development stack.
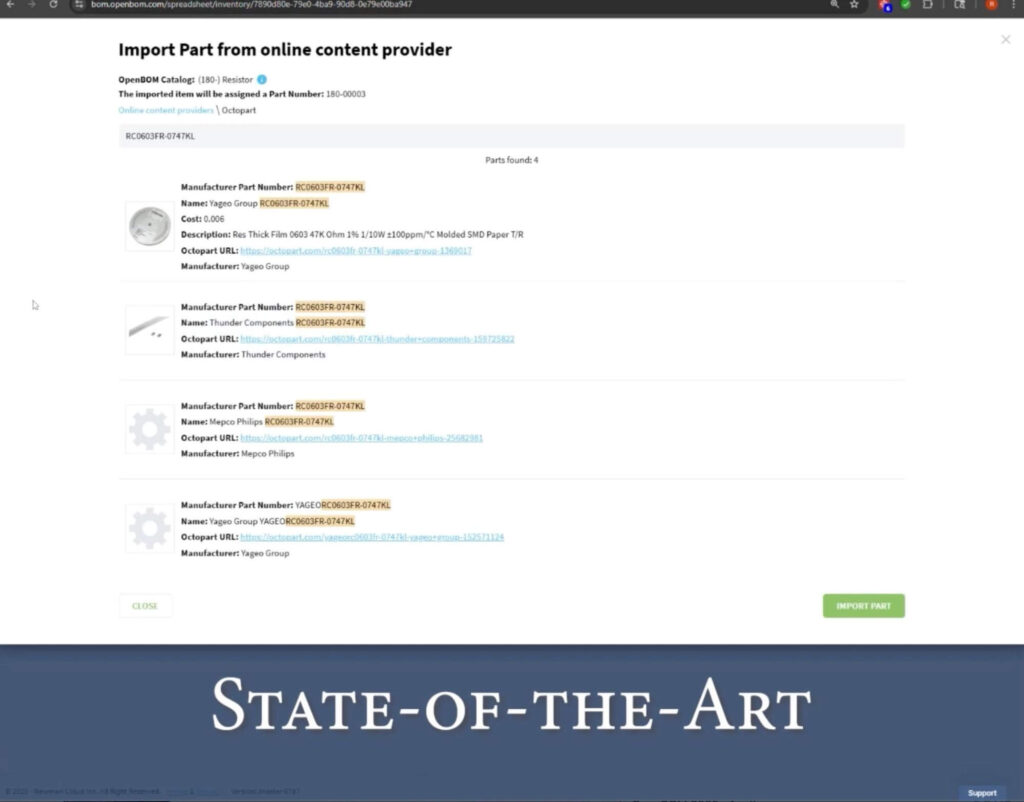
“OpenBOM is a state-of-the-art, modern PLM tool. It delivers the flexibility we need, it’s easy to use, and most importantly, it integrates and synchronizes our PLM data seamlessly with our design systems,” Richard explains.
Unlike older non-modern PLM systems, OpenBOM is built to work with the tools engineers already use. For Abram Scientific, this meant a direct connection between CAD and OpenBOM—allowing design data to flow automatically into the BOM and accessing data.
Connecting Design into the Full Product Development Ecosystem
But the benefits didn’t stop with CAD.
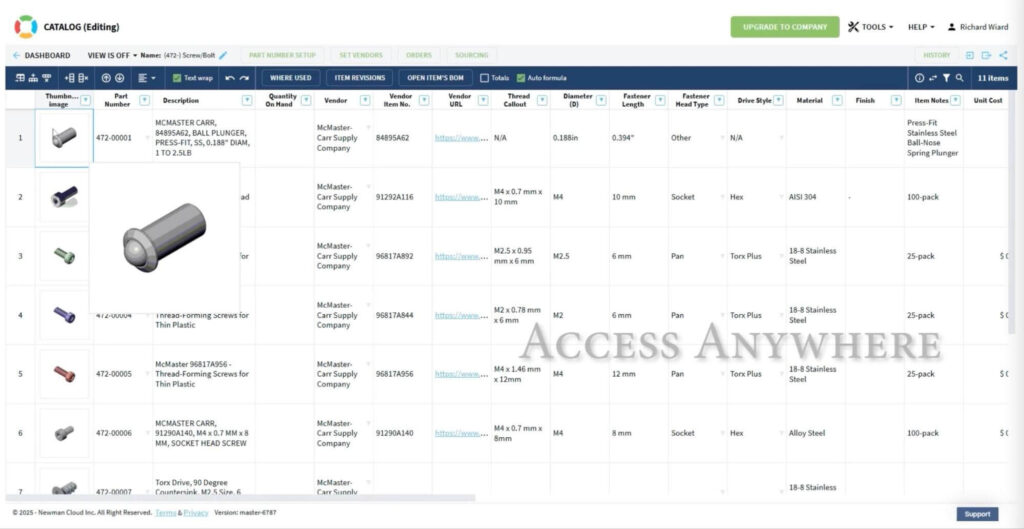
OpenBOM became a central hub that connects engineering design with requirements and quality management systems. This integration is especially critical in the medical device industry, where compliance, traceability, and validation are non-negotiable.
Why OpenBOM Stands Out for Medical Startups
✅ Centralized, Cloud-Native Access
With OpenBOM, our product data lives in the cloud—secure, centralized, and always up-to-date. Our entire team, from design to procurement, accesses the same information in real time, wherever they are.
✅ Advanced Revision and File Management
Using OpenBOM’s Workspace Manager, we manage every file and revision with traceability and control. For regulated medical products, that’s essential. It allows us to maintain design history files (DHF), device master records (DMR), and ensure traceability across iterations.
✅ Seamless Integration with Engineering Tools
OpenBOM integrates with all major MCAD and ECAD platforms. This lets us connect our mechanical, electrical, and system-level designs into one digital thread—something previously only available to enterprise teams with massive budgets.
✅ Affordable and Scalable
Unlike traditional PLM platforms, OpenBOM doesn’t require long contracts, expensive consultants, or months of implementation. It’s a SaaS model that scales with us, making enterprise-grade capability accessible to startups like ours.
“The ability to connect our design environment with our engineering and quality tools is what really makes OpenBOM valuable,” says Richard. “It’s not just about BOMs—it’s about enabling a connected, collaborative process managing files using OpenBOM workspace manager and capturing BOMs directly from design.”
Early-Stage Strategy: Build for the Future, Start with Data
Abram Scientific took a proactive approach. Rather than waiting for complexity to pile up, they focused early on establishing a robust digital foundation for product development.
That foundation—anchored by OpenBOM—is what allows them to scale with confidence, maintain control of product information, and meet the strict requirements of regulated medical development.
“We integrated OpenBOM with our design and QMS/requirements tools early in the process. That decision is paying off now as we grow and move toward production,” says Richard.
Watch the Full Story
Listen to Richard Wiard, VP of Engineering at Abram Scientific, share how OpenBOM helps the company stay ahead of complexity and build a connected product development backbone.
Watch the video
Conclusion: Data is the Foundation for Medical Innovation
For Abram Scientific, OpenBOM has been more than a platform—it’s become part of how we innovate. From managing complex assemblies to enabling rapid iteration, it empowers our engineers and stakeholders to collaborate with confidence.
If your startup is building complex products, especially in the regulated world of medical devices, don’t settle for outdated solutions. Modern product development demands modern infrastructure. OpenBOM is that infrastructure.
With OpenBOM, Abram Scientific has created a scalable, collaborative, and tightly integrated engineering and production environment. The ability to connect design, requirements, and quality in a single, flexible PLM platform gives them the agility to innovate faster and deliver with confidence.
Data matters—and OpenBOM makes it work for you.
Learn more about Abram Scientific → https://www.abramscientific.com/
Learn more about OpenBOM → https://www.openbom.com
Join our newsletter to receive a weekly portion of news, articles, and tips about OpenBOM and our community.
